HazSite Tables and Fields for the Electronic Data Submittal Application-Version 7 (EDSA7)
This document explains EDSA7 on a field-by-field basis. New fields or changes to the fields have been highlighted in Bold text. The component tables and fields are detailed below. By way of a general discussion of the tables and fields, it should be noted that EDSA7 (like all prior versions) reviews tabular data by field position. You will note that the first column in the description of the tables (below) contains the field position. Because EDSA programs check data by column position, omitting a column can often result in thousands of errors! It is critically important that all columns be retained in each table, even if no data are to be stored in the column. While NJDEP attempted to use simple, meaningful names for the columns, many end-users have had (and continue to have) misconceptions about some of the columns. We have attempted to clarify names in the EDSA error messages as well as in this webpage.
Different colored rows correspond to different requirements. Those details as well as the field datatypes can be found in the EDI Manual.
Back to Top
Back to Top
HZRESULT
Back to Top
DTST
- Directory
This field is limited to 8 characters. It is intended to allow the submitter to create unique identifier for the submission. It is also used as part of the set of key fields used to distinguish one EDD from another in NJDEP's catalog, therefore it is advantageous to use a unique name. As an example, if quarterly ground water monitoring is being conducted at a site, you could name the submission's directories 2012QTR1, 2012QTR2, etc
- Desc
The description of the dataset is intended to convey information about the site (or "Program Interest" or Case) that would help to identify the location of the sampling in the event that the SRPID or other information contained in the various files is inadequate for identification purposes. While interpretation of this information is fairly broad-based, as to what is acceptable, certain entries (e.g. "GW") are not acceptable.
- SRPID
The SRPID is intended to be a unique identifier of a case. It can take many forms, based on historical identifiers used by the Site Remediation Program. Examples are shown below.
| Type |
SRPID Example |
| Preferred ID (AKA PI ID) |
212005 |
| Preferred ID (AKA PI ID, GEDI ID) |
G000002254 |
| ISRA (ECRA) Case Number (<2000) |
E84002 |
| ISRA (ECRA) Case Number (>2000) |
E20050211 |
| Incident Number (AKA Communication Center Number) |
02-04-15-1022-15 |
| Incident Number (without hyphens & seconds) |
8902280959 |
| EPA ID Number (Superfund, etc.) |
NJD980761365 |
| EPAID Number (aka NJ Known Contaminated Site) |
NJL000070276 |
| TMS (Tank Management Section) Number |
N98-1505 |
| TMS (Tank Management Section) Number |
C94-1344 |
| Memorandum of Agreement (MOA) Case |
E20050211-M01 |
| UST Registration Number |
0093611 |
| Bureau of Field Operations (BFO) Case Number |
93-06-15-SP01M |
| Publicly Funded Case Number |
79-10-17-02 |
| Responsible Party Case Number |
M175393 |
- Consultant
This field is for the name of the primary consulting firm that is collecting samples and compiling reports.
- Phase
This is not a required field, but can be used to track which phase of investigation/remediation is represented by the accompanying dataset
- Status
This field is a legacy field that is not used anymore. However, the column is still needed for table format checks. DO NOT delete the column from the DTST table.
- Transmit
This field is a legacy field that is not used anymore. However, the column is still needed for table format checks. DO NOT delete the column from the DTST table.
- Submitdate
This field indicates the date that the EDD was originally submitted to NJDEP. If a replacement dataset (intended to overwrite a failed dataset) is being submitted, it is especially critical that this date not be changed, so that the original can be located, as this field is part of the Primary Key for the DTST table
- Packnum
This field is a legacy field that is not used anymore. However, the column is still needed for table format checks. DO NOT delete the column from the DTST table.
- Contactnam
This field is for the name of the data preparer producing the EDD or a person with full knowledge of how the data was prepared.
- Contacttel
This field is for the Contact's phone number.
- Contactext
This field is for the Contact's phone extension number.
- Contactema
This field is for the Contact's email address.
CONTACTNAM (Contact Name), CONTACTTEL (Contact Telephone Number), CONTACTEXT (Contact Phone Extension Number) and CONTACTEMA (Contact E-mail) are new fields required for electronic processing of the data. Since there are no more case managers conducting review of cases, contact information for EDDs must be readily available to anyone in NJDEP who may have to interact with the submitter of the EDD
HZSAMPLE
- SRPID
The record should match DTST SRPID & HZRESULT SRPID. see SRPID
- Sampdate
The date the sample was collected
- Sampnum
SAMPNUM is a field that (in conjunction with SAMPDATE) is intended to provide a unique link between one row in HZSAMPLE and related rows in HZRESULT. The field will accept any combination of up to 50 alpha-numeric characters, the combination of which must be used only once in HZSAMPLE. An example of an acceptable SAMPNUM might be something like: SB5-12(1.5-2.0); this combination is intended to indicate soil boring #12 from AOC 5 at a depth of 1.5 to 2.0 feet below the ground surface. The corresponding FIELDID would be SB5-12. Since the FIELDID is intended to indicate the unique location of a sample (in terms of its X-Y coordinate), a second sample from SB5-12 might have a SAMPNUM like SB5-12(3.0-3.5). If one used these two strings as FIELDIDs, an error would be generated for having two DIFFERENT FIELDIDs for the same coordinate. One FIELDID must have one unique coordinate, which makes the selection of FIELDIDs and SAMPNUMs across EDDs important.
Refer to Primary Keys for a further explanation of how SRP uses the SAMPNUM and SAMPDATE fields to enforce uniqueness between the HZSAMPLE and HZRESULT tables.
- Samptime
The sample time field is 25 characters in length, increased from 5.
The expanded field allows for a timestamp format: "MM/DD/YYYY hh:mm" Example: 12/04/2009 13:55
5-character time as a 24-hour variant (military time): "hh:mm" remains acceptable. Example: 10:22 or 15:17
Date/Time Format Error Messages:
Not a valid date. The most common problem with date formats occurs when a date is converted to a numeric expression ... DATE = 36443.54167 - normally, this error is associated with the Lotus 123 (WK1 format) which uses a standard format for date that is stored (for example) as 36443.54167. This number represents the elapsed days from January 1, 1900 plus a fraction representing the time of day. It equates to 10/12/1999 13:00. The format can be interpreted, but by many end-users mistake the fact that the database fields require a maximum length of 8 to include the date separators, whereas the databases actually only count numbers. Therefore, 10/15/01 takes up only 6 of the 8 characters in the database. XL will interpret this as 10/15/1901 if it is not entered 10/15/2001 in the original dataset.
If using the 5-character format, the SAMPTIME field for time-of-day in 24-hour format should contain no date.
Minutes in time-of-day should be less than 60 - this follows the basic format but might be written 12:75. Since there are only 60 minutes in an hour, the 75 after the colon cannot be interpreted with certainty as to the author's intent.
Number in SAMPTIME not recognized as a time of day
Time-of-day field should not include non-numeric date information
Not a time in HH:MM 24-hour format
String in SAMPTIME not recognized as a time of day
SAMPTIME exceeds the 25-character limit
Time formats separated from the date have been submitted as .1109.5 - by multiplying this numeric expression by 24 hours in the day, it results in an expression for HH:MM:SS that evaluates to 02:39:46 and should be more correctly expressed as date plus 02:40 (e.g. 06/15/2010 02:40).
- DupSamp
The duplicate sample flag requires a one-character "Y" or "N" for Yes or No. Quality Assurance/Quality Control (QA/QC) requirements for laboratory compliance require periodic sampling of duplicates to ensure reproducibility of results on laboratory instruments. These results are to be reported, but for some purposes in reporting may be treated differently from original results. It is therefore mandatory to flag the duplicates in reporting. The main sample, from which a duplicate is collected, should be flagged with "N".
- Matrix
Click here for Matrix Valid Values
- Fieldid
FIELDID is a field that represents the SINGLE, UNIQUE location for a sample collected at a site. One FIELDID is permitted to represent one and only one location at a site. As such, keeping track of FIELDIDs and their associated X and Y coordinates at a site is absolutely critical to a proper understanding of all aspects of a cleanup.
One particularly difficult area is that of Monitor Wells. Because wells are repetitively sampled and older sites may undergo many changes of consultants, many of the wells installed have been re-surveyed multiple times and well names may have been entered differently between submissions of various EDDs. As an example, “MW-1” is not equivalent to “MW 1” or “MW01” for a computer that uses only the exact text string as a unique identifier. In order to correct these problems, SRP has begun collecting the Well Permit Number for each permitted well as a part of the EDD.
To request a list of locations (LocList) for a site, contact Henry Kindervatter by e-mail (henry.kindervater@dep.nj.gov). Include the Preferred ID (also known as the PI ID) of the case in question in the subject line of the e-mail.
- Aocid
The AOCID is intended to allow the evaluation of HazSite data by individual Areas of Concern (AOCs). This field can be used to tie into any identically-named AOC found in NJEMS. If the AOC is found in NJEMS, details related to the AOC can be linked to the HazSite data. While this is not a required field, it should be useful to the Responsible Party to demonstrate that certain areas of a site have been completed (or partially completed), possibly decreasing the potential Remedial Priority System (RPS) score for the site.
One of the potential uses for AOCs with additional information is to determine whether particular AOCs might be excluded (or partially excluded) from consideration in the RPS. Details from the New Jersey Environmental Management System (NJEMS) indicate that an AOC has been excavated to a particular depth. If samples can be tied to this AOC by AOCID, they would be excluded from consideration in RPS scoring because they have been excavated.
Figure 2:
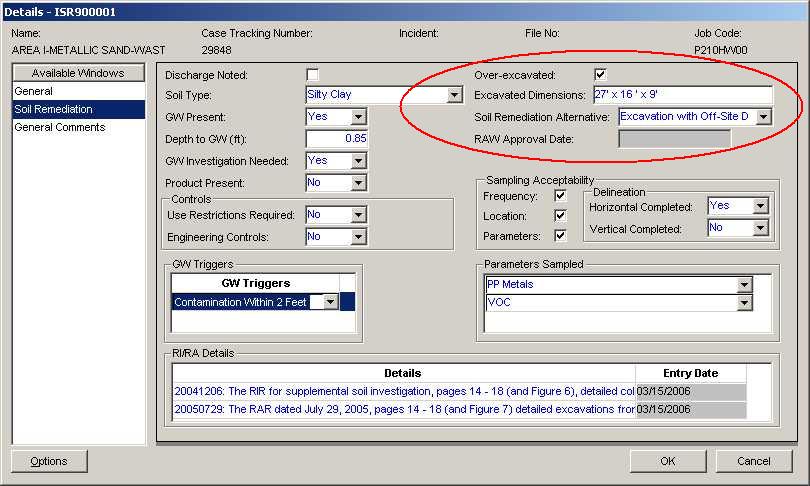
-
Lat_degree,
Lat_minute,
Lat_second,
Lon_degree,
Lon_minute,
Lon_second
The Site Remediation Program prefers that EDDs have their X-Y coordinates submitted in State Plane Coordinates (SP_X and SP_Y), using the North American Datum 1983 (NAD83) standard and expressed in feet. For convenience to the person(s) submitting EDDs, Latitude and Longitude are still being supported. It is, however, recommended that either State Plane coordinates OR Latitude and Longitude be submitted and NOT BOTH. If both formats are submitted, EDSA will generate an error if the points are not within two feet of one-another.
In general, Latitude, Longitude and State Plane coordinates have been one of the most problematic aspect of the HazSite data collection effort. The old format for Latitude and Longitude (see tables below) required Degrees, Minutes and Decimal Seconds to be separated into their respective fields. Many problems appear to have arisen from users parsing Decimal Latitudes and Longitudes into the fields that are intended to hold Degrees, Minutes and Seconds - for instance, see the example below.
Take for example the location for NJDEP in Trenton: The Latitude and Longitude are: 40° 13' 14.49"N and 74° 45' 26.50"W respectively. Conversion to decimal Latitude and Longitude gives: 40.220691666666670, 74.75736111111111. If the end-user attempts to fit this into the old format, the result will be Latitude = 40° 22' 06.917" and Longitude = 74° 75' 73.111" respectively. EDSA7 will issue an error message informing the user that Minutes and Seconds are limited to 60. If the Longitude is changed from 74° 75' 70.8" to 75° 16' 10.8" (carrying 1 from Seconds to Minutes and from Minutes to Degrees), the resulting point plots in Perkasie, PA. For this particular case, EDSA would recognize two errors (Minutes > 60 and the coordinate is outside of the NJ rectangle), however it is possible to generate a point with neither of these errors that is still incorrect with respect to the actual location within the state.
Column specifications for the Latitude and Longitude fields have been changed to accommodate Decimal Degree format, Degree + Decimal Minute in addition to Degree + Minute + Decimal Second format (same as previously required). The currently-accepted format allows for the following variations:
Degrees, Minutes and Seconds Latitude: 40° 13' 14.2", Longitude = 74° 45' 25.5"
Decimal Degrees: Latitude = 40.22061, Longitude = 74.75708
Degrees, Decimal Minutes: Latitude = 40° 13.004, Longitude = 74° 45.007
If a submission is to be made in a Decimal Latitude and Longitude (or Decimal Minutes format), both coordinates must be submitted for the row in the same format and the COORDMETH field MUST contain "DECIMAL DEGREES" (or "DECIMAL MINUTES") in order for EDSA to recognize the format. Go to HZSAMPLE table
Old Format
| Field |
Type |
Length |
Decimals |
| LAT_DEGREE |
Numeric |
2 |
|
| LAT_MINUTE |
Numeric |
2 |
|
| LAT_SECOND |
Numeric |
7 |
4 |
| LON_DEGREE |
Numeric |
2 |
|
| LON_MINUTE |
Numeric |
2 |
|
| LON_SECOND |
Numeric |
7 |
4 |
| SP_X |
Character |
14 |
|
| SP_Y |
Character |
14 |
|
New Format
| Field |
Type |
Length |
Decimals |
| LAT_DEGREE |
Numeric |
10 |
7 |
| LAT_MINUTE |
Numeric |
8 |
5 |
| LAT_SECOND |
Numeric |
6 |
3 |
| LON_DEGREE |
Numeric |
10 |
7 |
| LON_MINUTE |
Numeric |
8 |
5 |
| LON_SECOND |
Numeric |
6 |
3 |
| SP_X |
Character |
14 |
|
| SP_Y |
Character |
14 |
|
- Sp_x, Sp_y
The HazSite SP_X and SP_Y fields should be used to record NJ State Plane Coordinate System (SPCS) x and y coordinates for all sample location. Although it is impossible to map a curved Earth as a flat map using a plane coordinate system without distorting angles, distances, or areas, it is possible to design a map projection such that these three criteria are undisturbed or minimally distorted. Many states use a State Plane Coordinate System (SPCS) which is a map projection system that minimizes angular distortions if only a small portion of the Earth is flattened out (i.e. the State of New Jersey). Thus, the New Jersey SPCS is a rectangular (x, y or easting, northing) coordinate system describing geodetic positions, for New Jersey, on a plane. The NJSPCS's x,y coordinates are computed by projecting latitudes and longitudes from a mathematical approximation of the earth (i.e., NAD83) onto a rectangular grid. The x,y origin of the NJSPCS grid is at latitude 38° 50' N and longitude 74° 30' W. Due to this origin all NJSPCS coordinate values that fall within NJ are positive numbers (see diagram).This origin translates in NJSPCS, NAD83, US feet units to roughly 492,125 (easting), 0 (northing).
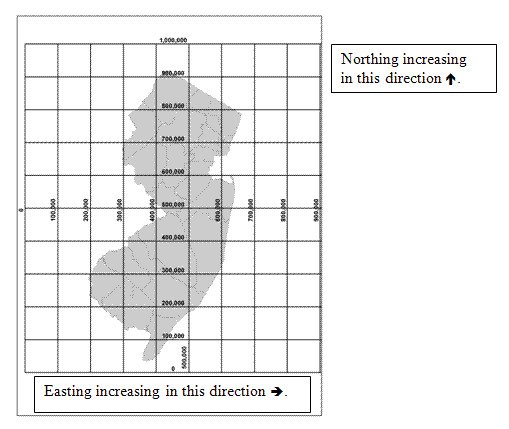
There are many latitude and longitude converters on the internet if latitude and longitude are the only values available to you. SRP requests that all SP_X and SP_Y values be recorded in NJSPCS in US feet units, referenced to the NAD83 horizontal datum. SRP expects an accuracy range of <10 feet.
- Depth_top
This field previously was used to track soil and ground water depths. Beginning on November 15, 2012 this field tracks soil samples only. This field is measured in feet, using a text field with a maximum of 2 places after the decimal point. Since the decimal place takes up one of the six characters, that leaves three characters to the left of the decimal and two to the right or else four characters to the left of the decimal point and one to the right (i.e. format is either 999.75 or 9999.5)
DEPTH_TOP is measured from the ground surface to the top of the sample.
- Depth_botm
Required only for Soil sampling. This field is measured in feet, with a maximum of 2 places after the decimal point. It is used to indicate the endpoint of the soil boring segment that was collected for the sample in question.
- GroundElev
The Ground Elevation is an absolute value for the Z-Coordinate, measured in feet above mean sea level (MSL), with a maximum of 2 places after the decimal point (as with DEPTH_TOP above). It is used to indicate the ground surface elevation for the sample in question. For wells, this point requires certification by a licensed surveyor. The surveyed point for a well, if known, can be used to measure other points on the site to approximate their locations relative to the surveyed point.
- Well_elev
The Well Elevation is a value for the Z-Coordinate, measured in feet above mean sea level (MSL), for the location of the surveyor's mark on the well casing. This measurement should be surveyed to the North American Vertical Datum (NAVD) 1988, to an accuracy of 0.2 feet, using generally accepted surveying methods.
- Samptype
A required field with valid values (listed in the table below). Errors for this field often come from misspellings of the acceptable values. Please also note that the use of "OTHER" in this field requires an explanatory note in the SAMPNOTE field.
- Datetolab
Date the sample was delivered to the laboratory for analysis. Required format is MM/DD/YYYY.
If more than one Sample Delivery Group (SDG) is associated with a single field sampling event, separate record rows with associated changes in SAMPNUM should be employed if delivery dates to the lab (or labs) differ between groups.
- Sampmethod
This field is used to track the sampling method used in collecting the sample. It typically includes entries such as "Bailer", "Split Spoon", "Low-Flow Pump", "Hand Auger", etc.
Any errors encountered from this field have in the past been due to exceeding the 15-character allowable length for the field.
- Sampnote
This field is intended to be used for further explanation of "OTHER" in the MATRIX and SAMPTYPE fields. Any notes related to the row in the sample file can be stored here, however.
- Submitdate
This field in the HZSAMPLE table does not need to be populated because SRP now relies solely on the SUBMITDATE in the DTST file. However, this column is still needed for table format checks. DO NOT delete the column from the HZSAMPLE table.
- Qaqc
This field is used only for NJDEP's internal purposes. However, this column is still needed for table format checks. DO NOT delete the column from the HZSAMPLE table.
- Coordmeth
This new field tracks the coordinate method. It is only used when submitting files in either of two newly-allowed Lat/Long formats and must appear EXACTLY as one of the following: "DECIMAL DEGREES" or "DECIMAL MINUTES"
- Coordnote
This new field allows additional information related to coordinate collection to be stored.
- GWDepthPri
This field measures the depth to ground water before purging a well. It is reported as the absolute value of feet below the ground surface (NOTE: GROUNDELEV the measure of ground surface is measured in feet above mean sea level). This new field is now a required field.
- GWDepthPos
This field measures the depth to ground water after purging a well. It is reported as the absolute value of feet below the ground surface (NOTE: GROUNDELEV the measure of ground surface is measured in feet above mean sea level). This new field is now a required field.
- Screentop
This field measures the depth, in absolute feet, from the ground surface to the top of the screen (or packer interval or passive diffusion bag). This new field is now a required field. NOTE: GROUNDELEV is measured in feet above sea level.
- Screenbot
This field measures the depth, in absolute feet, from the ground surface to the bottom of the screen (or packer interval or passive diffusion bag). This new field is now a required field. NOTE: GROUNDELEV is measured in feet above sea level.
- Wellpermit
This is now a required field. The well permit number is the unique identifying number generated by NJDEP’s Water Allocation, Well Permitting group. Prior to 2008, the well permit was based on the Atlas Grid system. The Atlas Grid system divides the state of NJ into “Atlas Pages”, which, in turn, are subdivided into smaller rectangles. These blocks are further sub-divided into smaller rectangles known as Atlas Cells. There are over 150,000 cells in NJ. The first two digits of the well permit number represented the Atlas Grid cell in which the well was located (Note that in Figure 5 the first 2 digits in the Application Number are the same as the first two digits in the Location, or Atlas Grid). The remaining digits represented the sequential number for the well drilled in that Atlas Grid. NJDEP eventually found it necessary to "Normalize" the data for wells to help with lookups of wells when the paper information was entered into databases. To do so, the well had "leading" zeros placed in front of the well number. Originally, normalized wells were stored as Atlas Grid + hyphen + Well Number (26-01335, for instance). All wells prior to 2008 have now been changed to a 10-character text file containing only numbers. The first two continue as the Atlas Grid; the sequential number of the well within the Grid Cell remains, but the remaining characters needed between these two numbers are filled with zeros, the result of which is a 10-character "number" in a text format.
In 2008, a computerized system replaced the older hand-entry of wells. Some information continued to come in as paper copies and had to be hand-entered. The system for tracking these wells was changed to have a "P" for paper or "E" for electronic (uploaded via the internet), followed by 4 characters for the year and then 5 characters that denote the sequential number of the well for that year. For example, E200900587 would be the 587th well reported for 2009 (and we also know that it was submitted electronically).
To find unknown Well Permit Numbers, run the following Data Miner Report:
Select XY Permit Well Search (the half-mile radius is recommended to start)
This report returns permits, records and decommissioning within the specified search radius. Select and copy the contents of the HTML table into a spreadsheet and filter for the appropriate location(s). To do this, it is important to note that the Document, Well Use and Physical Address are the most important fields. It is recommended that Well Use be filtered to Monitoring and Monitoring Replacement (and any other relevant types). Next, the Document can be filtered to Record (looking for wells that were actually installed). If the address or block(s) and lot(s) are known, use of filters on these fields can be very helpful. Using a combination of address, block, lot and Well Name, it should be possible to find Permit numbers related to site wells. For help with this query, contact Henry J Kindervatter or by phone (609) 633-1419.
- Srp_dir
This new field is not a required field. It will be used by NJDEP when returning datasets. When returning datasets to the responsible party, SRP will often concatenate multiple datasets into a single set of HZSAMPLE and HZRESULT files. In order to detail the source rows of data, SRP indicates the dataset (by catalog number) from which the data were derived. This will become necessary more often as SRP implements corrections to the X-Y coordinates and FIELDIDs that are required for other Department initiatives.
- Samplabid
The SAMPLABID is a secondary key between the HZSAMPLE and HZRESULT tables using the LABID. It is a newly-required field. It provides a double-check on the primary key and provides a mechanism for tracking samples that have been diluted in the laboratory analysis process. One of the biggest sources of errors prior to EDSA7 occurs when a laboratory runs one or more serial dilution(s) of samples that have high concentrations of target analytes. If a new row is not created in the HZSAMPLE table to accomodate these dilutions, the one-to-one relationship (see primary key) between HZSAMPLE and HZRESULT is broken, resulting in difficulty determining the proper set of results to use for analyzing the sampling episode.
HZRESULT
- SRPID
The record should match DTST SRPID & HZSAMPLE SRPID. see SRPID
- Sampdate
The record should match HZSAMPLE SRPID. see SAMPDATE
- Sampnum
The record should match HZSAMPLE SAMPNUM. see SAMPNUM
- Labid
This is the identification number given to the specific sample by the laboratory. This will also be referred to as SAMPLABID. This is a required field and cannot be left blank. The field is being checked in EDSA7 to enforce a 1-to-1 link to HZSAMPLE. When multiple Sample Delivery Groups (SDGs) are required, the LABID must be configured to correspond to the group submitted. This requires separate SAMPNUMs for each group.
- Tdanalyz
This required field has been changed from the DANALYZ (Date Analyzed) field in the older version of EDSA to a Time-Date Analyzed field. The current field accepts MM-DD-YYYY HH:MM or translatable variants of that format. The old field accepted dates in the MM-DD-YYYY format.
- Labname
This field is required if a NJ lab certification number is not available.
- Njdlabcert
This field is required and is based on NJDEP's Office of Quality Assurance certification number for laboratories. Each participating laboratory in NJ has a unique 5-digit certification number. Out-of-state laboratories may have a longer number with a string designating the location (State, Province, etc), therefore this is a text field. If no laboratory certification number is available, the LABNAME field becomes the required field for laboratory identification. Where possible, laboratory names should be spelled out (avoid abbreviations).
- Resulttype
The Result Type is an abbreviation for the types of results that can be handled by EDSA (see the RESULTTYPE valid values)
- Analtparam, Cas
The ANALTPARAM field is used for the storage of Analytes, Parameters, TICs, Surrogates and Internal Standards. This is a required field. In the past, NJDEP required this field, but not the Chemical Abstracts Service (CAS) number as a required field. The CAS requirement was subsequently changed so that the CAS field is also required.
In EDSA7, these fields are both required. The original rationale for excluding CAS numbers from the submission requirement in earlier versions of EDSA was that computing power was insufficient to support the lookup of huge numbers of possible combinations of CAS and ANALTPARAM. Because the computer limitations have been largely eliminated, CAS/ANALTPARAM combinations will be published and strictly enforced. Chemical Abstracts Service supports a web site for CAS Number Lookups. Additionally, EDSA7 stores a lookup table in your EDSA installation directory RESLTYP4.DBF (default installation directory is: C:\Program Files\EDSA\ or C:\Program Files(x86)\EDSA\) as a dBase file for use in looking up acceptable combinations of CAS and ANALTPARAM. This table is a valuable resource to help avoid potential errors between these two fields.
Common Classes of CAS and ANALTPARAM errors:
- Invalid Check-Digit - CAS web site that explains the structure of a CAS number
- Missing or Misplaced Hyphens - CAS numbers consist of a series of up to 10 total integers separated by 2 hyphens: 2 to 7 integers + a hyphen + 2 integers + a hyphen + 1 integer
- Decimals in CAS Number - There are no decimals in CAS numbers, only Integers
- Alphabetic Characters in CAS Number - There are no alphabetic characters in CAS numbers, only Integers
- Invalid (non-numeric) CAS - There are no characters apart from integers 0 through 9 and the hyphen (-) in CAS numbers
- Too few Digits in CAS - The first of three parts of the CAS number can have from 2 to 7 integers; the second part has 2 integers and the third part has 1 integer
- Required ANALTPARAM is empty - ANALTPARAM is a required field - an empty field will always generate an error for this field
- ANALTPARAM exceeds 60-character limit - ANALTPARAM has a 60-character limit; characters will be truncated on the right if that limit is exceeded
- Filtunfilt
FILTUNFILT is now a required field. There are two possible valid values for this field. They are: F for filtered samples and U for unfiltered samples. Filtered samples are not routinely accepted by NJDEP, therefore in order for filtered samples to be used in the decision-making process at a site, a variance from the Technical Regulations would be required. If filtered samples are collected, a separate row should be added to the HZSAMPLE table do differentiate the filtered results from the unfiltered results (assuming unfiltered results are also reported).
- Conc
The concentration field is mandatory. Text-string numeric equivalents for concentrations of Analytes, Parameters and TICs are stored here. Soils are to be reported in parts per million or milligrams per kilogram and for water parts per billion or micrograms per liter are to be used. For those samples that do not have a detectable result, use 0. This field is translated for many purposes into a numerical expression, therefore the use of ND (for Non-Detect) is inappropriate. Non-numeric expressions are to be reserved for the QAQUAL (QA Qualifier) field. The use of 0 allows analytical programs that review data to definitively determine that there was no detection for the sample. The variants of the minimum detection limit in the MDL or QUANTTYPE and QUANTLEVEL fields indicate reporting and detection limits for the analysis and are the appropriate place to put such data. This field is not for use with various qualifiers (i.e. not for "U" or "<" or ">").
- Concunits
The CONCUNITS field tracks concentration units. If entering the value for another type of parameter, enter the appropriate units, or if no unit of measure is applicable (a dimensionless parameter, for example), you may enter N/A. Site Remediation no longer asks for ug/Kg and ug/L to be generalized to PPB or for mg/Kg and mg/L to be generalized to PPM. Knowing whether the basis is mass or liquid volume is helpful to reviewers and users of the database.
Please use the Latin letter u instead of μ (Greek lowercase letter mu also called the micro sign). This symbol is not among the standard printable ASCII characters 32 through 127, so it is displayed differently from one font to another. Also a different character code may replace it as the information migrates from application to application. Therefore instead of reporting "μg/L" for micrograms per liter one should report it as "ug/L" in order to ensure that the units are interpreted correctly. Likewise the non-ASCII degree symbol should not appear in a temperature unit, which can be "C" or "deg C" or "F" or "deg F". Accordingly the customary unit of electrical conductance in an aqueous solution microSiemens per centimeter can be "uS/cm" or "umho/cm" or "umhos/cm".
For more information see NJrefVV1.MDB within the EDSA directory (default installation directory: C:\Program Files\EDSA\ or C:\Program Files (x86)\EDSA\). That database has a table of recognized valid values for CONCUNITS.
- Qaqual
The QAQUAL, or Quality Assurance Qualifiers field stores information related to qualifiers to the Concentration field. The standard qualifiers listed below shall be used when appropriate (extracted from NJDEP laboratory services contract). The field is not restricted to one qualifier. If a laboratory specific qualifier is used, the qualifier must be fully defined in the SAMPNOTE field. For further information, contact your laboratory. If you represent the laboratory and wish to discuss qualifiers with NJDEP, contact Hazsite Help Desk hazsite@dep.nj.gov or call (609) 633-1380.
- Mdl
Method Detection Limit (MDL) The definition of MDL from the Field Sampling Procedures Manual - Glossary is "the minimum concentration of a contaminant that can be measured and reported with a 99% confidence that the analyte concentration is greater than zero and is determined from analysis of a sample in a given matrix containing the analyte".
- Quanttype
Quantitation Type (QUANTTYPE) is the lowest concentration, above background noise level, that an instrument can reliably detect. The value entered for QUANTTYPE (almost all of the time) will be the Reporting Limit.
Parameters do not require a RL.
- Quantlevel
The Quantitation Level (QUANTLEVEL) is the actual numeric value associated with the Quantitation Type.
- Anlys_mthd
Analysis Method identifies the analytical method used. The field must contain the method number/name preceded by the organization in which the test originated. If methods listed have been revised after the date of publication of the HazSite application and this manual, choose the most current version/update of the method. If entering data in this field for a common parameter and there is no applicable analytical method, add N/A. This has always been a required field, but it has not been rigorously enforced until this version of EDSA. EDSA7 stores a lookup table in the EDSA directory NJrefVV1.MDB (default installation directory: C:\Program Files\EDSA\ or C:\Program Files(x86)\EDSA\). That database has tables of recognized valid values for ANLYS_MTHD.
- QAQC
The QAQC field has been changed from the original One-character field to a 15-character field in order to accommodate a Sample Delivery Group (SDG) number.
NJDEP- SRP Low Level USEPA TO-15 Method (NJDEP-LLTO-15-3/2007) ... For a full explanation of the Sample Delivery Group, click on the preceding link and go to section L at the bottom of page 8 and onto the top of page 9.
- Uncor_conc, Uncor_unit
These fields were added to support the Indoor Air Sampling program.
UNCOR_CONC (or Uncorrected Concentration) is used for the raw concentration measured for air results, (measured in parts per billion volume = PPBV - see below) for the EPA TO-15 method.
UNCOR_UNIT (Uncorrected Units) is used for the raw units measured for air results (e.g. parts per billion volume = PPBV) for the EPA TO-15 method.
- Reten_time
RETEN_TIME is a new field that is now enforced for Tentatively Identified Compounds (TICs). This was formerly an alternative place to report the retention time for any result that has ResultType = "T". Retention time should be recorded to 1/100 of a minute.
- Dilut_fac
This field refers to the dilution of a sample that exceeded the range of calibration of a laboratory instrument and had to be re-run at a lower concentration. A portion of the remaining original sample would be diluted with distilled or deionized water at a particular ratio, for instance 10 to 1 (or 10:1). A 10:1 ratio would result in a dilution factor of 10 being recorded for each row in HZRESULT associated with the diluted sample (which must also have a different SAMPNUM and LABID from the original, undiluted sample). In addition, a separate parent row must be added to HZSAMPLE to account for the dilution and re-sampling of the sample that exceeded the instrument's range.
- Prep_mthd
This field indicates the preparation method used to prepare the sample(s) for analysis. Certain analyses require a preparation method. Among these are TCLP and SPLP analyses. For these and any others, where a preparation method was used, the laboratory shall list the method in this field.
- Clnup_mthd
Some samples exceed the calibration range of a laboratory instrument, due to interference from some non-target compound. Cleanup methods allow the offending compound to be neutralized, while allowing the target compound to be acceptably quantified. A re-run of the dataset would be required after applying a cleanup method, therefore rows that utilize a cleanup method would be subject to similar requirements as those that underwent dilution - namely, a separate set of rows would be created for the target group in HZRESULT as well as a new parent record in HZSAMPLE.
Back to Top
| PI |
Preliminary Investigation |
| SI |
Site Investigation |
| RI |
Remedial Investigation |
| RA |
Remedial Action |
| RAO |
Remedial Action Outcome |
| MP |
Monitoring Permit |
See EDI Manual
See EDI Manual
See EDI Manual
See EDI Manual
See EDI Manual
See EDI Manual
Back to Top
More details on SAMPNUM
The Primary Key is defined as one or more fields which, taken together, can be used to identify a single unique row in the Parent Table. The Parent Table in this case is the HZSAMPLE table. It contains information about samples collected in the field. A Child Table contains multiple rows (a One-to-Many relationship) of data that are related to the Parent Table. In this case, the HZRESULT table is the child table because it has many analytical results (one per row) that are related to a single row in the HZSAMPLE table. In the HZRESULT table, duplicate records include ANALTPARAM as a part of the primary key to the HZRESULT table. This means that results having more than one unique result row for a single SAMPNUM are counted as duplicates and an error counted for each row. A final requirement for key fields is that they cannot be empty (null).
Orphan Records: "Orphan Records" are records that have information in the Child Table with no matching records in the Parent Table. For instance, in the example in Figure 1 below, if any one of the three highlighted key fields were changed in HZSAMPLE (e.g. if SAMPDATE was changed to 2/28/2008) all of the related HZRESULT records would become "orphans" because they no longer correspond to the "parent" record. For more information about Parent and Child records see the section on the Primary Keys
No Results (Orphans) Error Message: No result records found for sample.
Explanation: Records in HZRESULT exist with no match in HZSAMPLE. This can be caused by a mismatch in the SAMPNUM or SAMPDATE fields between the two tables. If they do not match, Childless records (in HZSAMPLE) or Orphan records (in HZRESULT) may be found.
| SRPID |
Character |
24 |
| SAMPDATE |
Date |
8 |
| SAMPNUM |
Character |
50 |
The Electronic Data Submittal Application (EDSA) uses a three-field Primary Key: SRPID + SAMPDATE (as a character expression of the date) + SAMPNUM. These three fields exist in both the HZSAMPLE and the HZRESULT tables.
Figure 1:
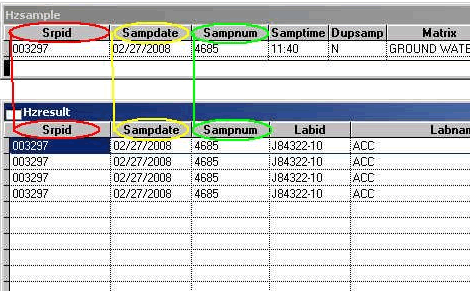
Back to Top
Error Message: Example: This SampLabId (new required field in HZSAMPLE that is related to LABID in HZRESULT) associated with multiple samples: 02/25/2008 195770 AND 02/25/2008 195771
Error Message: Analyte or parameter duplicates another that appears in 1 record(s) above. Each duplicate analysis of a sample requires a unique SAMPNUM.
Explanation: Please note (for the second error message) that there are 2 sets of the same analytes, Benzene, Ethylbenzene, Toluene and total Xylenes (with Benzene highlighted in red) for SAMPNUM = 1; The primary key uses SAMPDATE and SAMPNUM, therefore the two records are "duplicates". See Primary Key
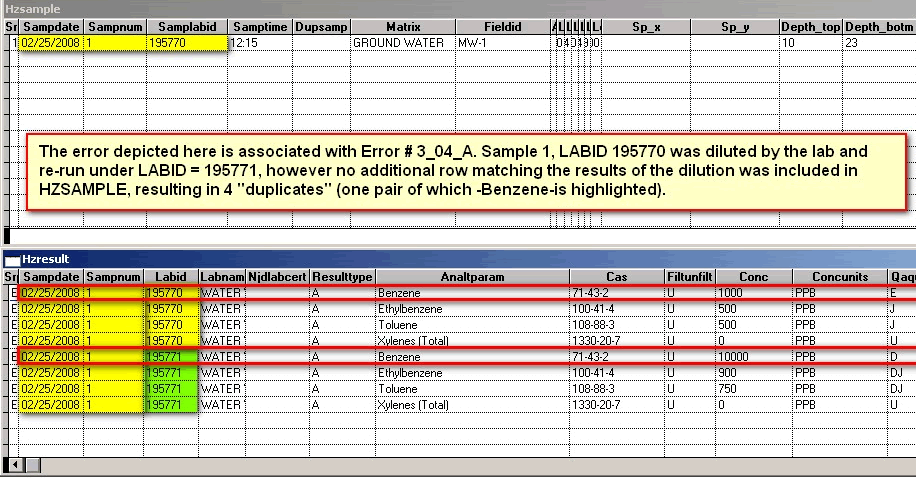
Corrected File; Duplicates remedied:
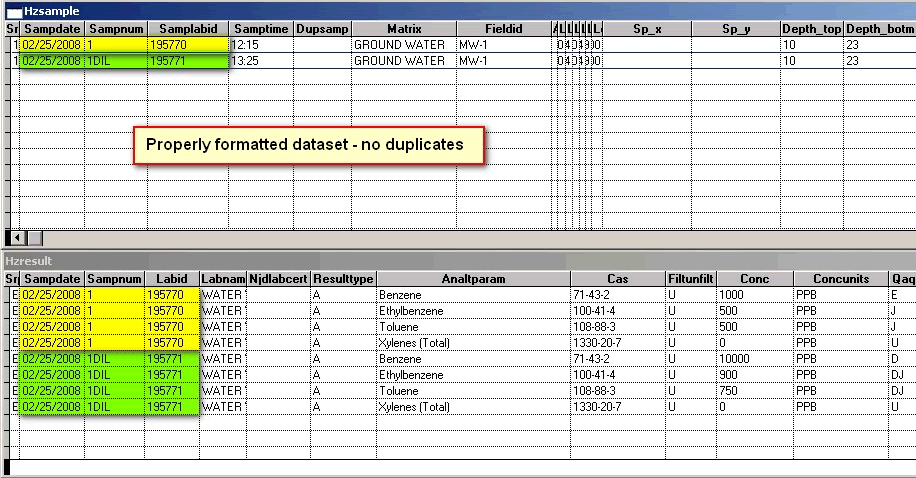
Back to Top
Error Message: Table structure problem. Missing at least 1 expected field.
Explanation: Missing columns will cause each succeeding column to be evaluated according to the rules for the column to its immediate left in the table. This will normally result in hundreds or even thousands of errors. If no data exist for a particular column (e.g. AOCID), leave the column in the table and input no data to that column.
Back to Top
Error Message: In DAO.Workspace err3274 - External table isn't in the expected format. Format differs from what file extension indicates. Opening file with Excel may help to confirm
Explanation: This error message occurs when one or more of the fields in HZSAMPLE or HZRESULT have are incorrectly structured or not in the proper order. Please check the table structure listed below to ensure the each field has the correct Field Name, is of the right type and that it conforms to the required size.
Back to Top
Error Message: An entire record of table is empty.
Explanation: EDSA encountered a row within a table (HZSAMPLE or HZRESULT) that contains ALL EMPTY FIELDS.
Back to Top
Error Message: Empty or corrupted table? RecordCount= 0 EOF= True.
Explanation: EDSA has encountered an entire table that has ALL BLANK ROWS (no data).
Back to Top
CHEMICAL NAME AND CAS NUMBER
CAS Lookup
Error Message Examples:
Error 3_08_A Example: NJDLabCert (AKA Lab Cert Num)= 12543; Anlys_Mthd= 200.7; ResultType= A; AnaltParam= COBALT; CAS= 1330-20-7; FiltUnfilt= WARNING 3_08_A Inconsistent name: This table reports different AnaltParam: CHROMIUM with same NJDLabCert (AKA Lab Cert Num), ResultType, CAS, FiltUnfilt, Anlys_Mthd
Explanation: The error above is an example of the same CAS number being used for both Cobalt and Chromium. This is an obvious error. NJDEP does not pick out the correct analyte based on CAS because it is not possible to tell whether the error was based on the wrong name and right CAS number or vice-versa.
Back to Top
Error Message: AnaltParam= Potassium; CAS= 9/7/7440; Anlys_Mthd= 200.7
Explanation: Excel converts Potassium's CAS # 7440-09-7 to a date format. If the cell in Microsoft's Excel program is set to its default value of "General" data, this CAS number is converted to a date format 9/7/7440. Other variants of the conversion may be 9/7/40 (which evaluates to 9/7/1940) or to 2023695, which is the serial date format.
How do I fix this error? The best remedy for this error is to set the default number format for all cells to "@" or "Text" on the "Style" sub-menu of the "Format" menu of Excel. Select the column of cells to format, then select Format -->> Style from the Excel menu. Choose Modify and modify the Number format to: Custom -->> @.
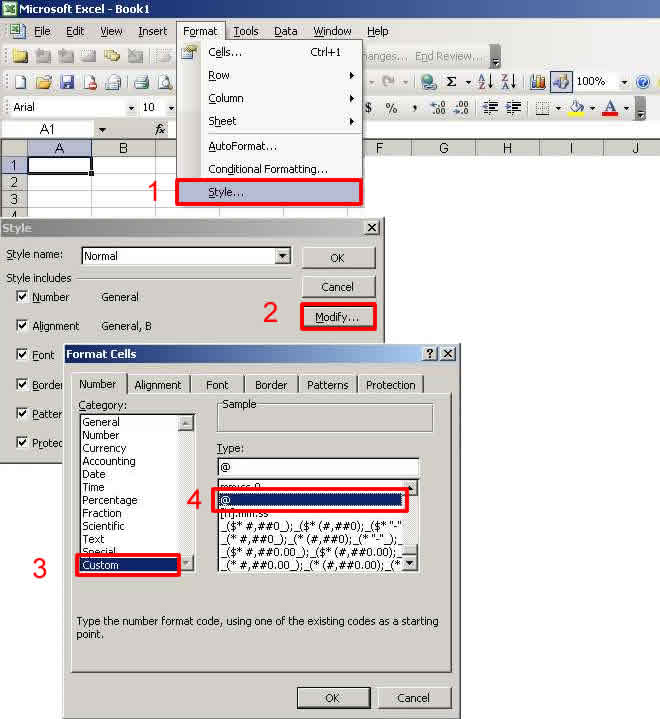
Back to Top
Error Message: All 9 nonblank samples share one coordinate location
Explanation: Each unique location must have a unique X and Y coordinate. A unique location is defined by the same FIELDID + X-coordinate + Y-coordinate for each distinct location that was collected in the field. There is now an absolute requirement for distinct X and Y locations that are submitted in either Latitude and Longitude or State Plane X and Y coordinates. Where both are submitted, they must agree as to the actual location of the sample. For further explanation, see FIELDID XY explanation
Back to Top
Error Message: Required DTST.SUBMITDATE is empty. EDSA attempts to check EDD without it.
Explanation: EDSA7 recognizes that the date of submission was omitted, but will check HZSAMPLE and HZRESULT for errors and regard the omission as a warning.
Back to Top
Error Message: SUBMITDATE, DATETOLAB, SAMPDATE later than file datetimestamp.
Explanation: The SUBMITDATE in HZSAMPLE should be earlier than the date stamp that indicates when the file was last modified (i.e. the file cannot be created before the sampling was conducted).
Back to Top
Error Message: SUBMITDATE, DATETOLAB, SAMPDATE [or TDANALYZ] later than today's date
Explanation: The date of sampling must be earlier than today's date. Each of these dates should be no later than the date when the checker is running. If one is later, please correct any typographical error in the date.
Back to Top
Error Message: SUBMITDATE, DATETOLAB, SAMPDATE or TDANALYZ earlier than 1950.
Explanation: The Site Remediation Program's Data requirements mostly began in the 1980's (1970's in some cases). Electronic Data Deliverable have been required since July 18, 1997. Submissions pre-dating these dates cannot be correct and may cause problems in the correct evaluation of progress in remediating a site, therefore, such dates will not be accepted.
If this error is found in the DTST.SUBMITDATE or HZSAMPLE.SUBMITDATE fields, it may be a problem that is inherent in the Lotus 123 format, which defaults all dates to the 1900's; dBase (DBF) files may also cause such errors because there is a system variable (CENTURY) that must be set to "ON" to prevent DBF's from defaulting to the 1900's.
Back to Top
Error Message:
- DATETOLAB earlier than SAMPDATE
- SAMPDATE later than DATETOLAB
- DANALYZ earlier than DTST.SAMPDATE
- DANALYZ Later than HZSAMPLE.SUBMITDATE
- DTST.SUBMITDATE later than HZSAMPLE.SAMPDATE
- DTST.SUBMITDATE later than HZRESULT.SAMPDATE
Explanation: The date order is checked, events happen in a specific chronological order.
| Order |
Table |
Field |
Explanation |
| 1 |
HZSAMPLE |
SAMPDATE |
Date of sampling (primary key field) |
| 1 |
HZRESULT |
SAMPDATE |
Date of sampling (primary key field) |
| 2 |
HZSAMPLE |
DATETOLAB |
Date samples were delivered to lab |
| 3 |
HZRESULT |
DANALYZ |
Date lab analyzed samples |
| 4 |
|
timestamp |
Date file was created |
| 5 |
DTST |
SUBMITDATE |
Date EDD was submitted to NJDEP |
Back to Top
Error Message: DANALYZ Earlier than SampDate in HZRESULT record OR DANALYZ Later than SubmitDate in DTST table
Explanation: The main error associated with the Date Analyzed Field is that this date cannot be earlier than either the sample date (SAMPDATE) or the Submission date (SUBMITDATE).
Error Message: DATETOLAB earlier than SAMPDATE
Error Message: SAMPDATE later than DATETOLAB
Explanation: Sampling date must occur before sending the samples to the lab.
Back to Top
DTST
HZSAMPLE
- Sampdate
See Date Errors
- DupSamp
Error Message: DUPSAMP valid values are Y or N
Error Message: DUPSAMP exceeds the 1-character limit
Explanation: Invalid Values - Native DBF formats use T for True or Yes and F for False or No. Microsoft Access uses 0 for False and negative 1 for True. These must be interpreted to the Y and N format. please note that EDSA7 only recognizes Y or N for this field.
- Matrix
All error messages encountered for matrix either have to do with something that is not in the Valid Values List or with "OTHER". Other is not accepted without an explanation provided in the SAMPNOTE field.
- Fieldid
Error Message: Example: This sample needs a FieldLocId (was FieldId) distinct from that of the sample in record 1, which has another SP_X (Easting); SP_Y (Northing) (584755.46140, 745017.03823)
Explanation: Two (or more) field locations are using the same X-Y coordinates. This field should be considered as the description of a location. The unique combination of a FIELDID + X-Coordinate + Y-Coordinate should denote a single boring in the field. Several samples may have been collected from this location, however if the FIELDIDs or the X-Y coordinates vary from this rule, errors will result. The most common errors of this type are generated when a consultant attempts to append the sample depth to the FIELDID, causing two (or more) FIELDIDs to have identical coordinates. EDSA7 indicates the row numbers from the HZSAMPLE table where the discrepancy is observed.
Back to Top
Error Message: Required FIELDID is empty.
Explanation: FIELDID is empty (null). EDSA has never allowed an empty FIELDID. This is a required field.
Back to Top
Error Message: FIELDID exceeds 20-character limit
Explanation: FIELDID exceeds the 20-character allowable limit for the size of this field. Formerly, EDSA permitted only 12 characters for the FIELDID, however EDSA7 has increased this to 20. Characters to the right of the 20-character limit will be truncated in NJDEP's version of the file.
Back to Top
Error Message: Same coordinates repeated for multiple location names (FIELDIDs)
Explanation: NJDEP requires one unique location (defined by the same FIELDID + X-coordinate + Y-coordinate) for each distinct location that was collected in the field. For wells there is a requirement to have a licensed surveyor verify the X, Y and Z coordinates to the nearest 100th of a foot. This information can be obtained from a Well Record document or from Site Remediation's Form B (Well Location Certification). For soil samples, one X-Y coordinate should be used for each boring conducted in the field. If the boring is to become a well, the Well's FIELEDID should be used to avoid having this error occur for two different FIELDIDs used for the same boring. One other common instance of this error occurs when the consultant attempts to append the depth of the sample to the FIELDID. HZSAMPLE already provides the DEPTH_TOP and DEPTH_BOTM fields for this purpose.
-
Lat_degree,
Lat_minute,
Lat_second
Lon_degree,
Lon_minute,
Lon_second
Error Message: All latitude and longitude fields must be populated if NJ State Plane Coordinates are missing.
Explanation: NJDEP requires a coordinate for each row in the HZSAMPLE table if the sample was collected, with the following exceptions:
- MATRIX = BLANK
- MATRIX = QC AIR
- MATRIX = QC SOIL
- MATRIX = QC WATER
- SAMPTYPE = BLANK
- SAMPTYPE = TEMP PILE
- For any other sample that does not have an X-Y coordinate, use "NO COORDINATE" in COORDMETHOD AND add an explanation to the HZSAMPLE.SAMPNOTE field
Back to Top
Error Message: West longitudes in NJ range from 73-54-07 to 75-33-22.
Explanation: In NJ, west longitude ranges from 73 54' 07" to 75 33' 22" - Decimal longitude ranges from 73.900 to 75.556. Entries in the corresponding fields must be within this range or they create an error.
Back to Top
Error Message: North latitudes in NJ range from 38-55-46 to 41-21-25.
Explanation: In NJ, north latitude ranges from 38 55' 46" to 41 21' 25" - Decimal latitude ranges from 38.930 to 41.357. Entries in the corresponding fields must be within this range or they create an error.
Back to Top
Error Message: Neither latitude/longitude nor state plane X/Y is provided
Explanation: Apart from the exceptions listed above, all rows in the HZSAMPLE table require coordinates in an acceptable form.
Back to Top
- Error Message: LAT_DEGREE (or LON_DEGREE) is non-numeric.
- Error Message: LAT_MINUTES (or LON_MINUTES) is non-numeric.
- Error Message: LAT_SECONDS (or LON_SECONDS) is non-numeric.
- Error Message: LAT_SECONDS (or LON_SECONDS) must be integer. Multiply fractional part by 60 to obtain decimal seconds.
- Error Message: LAT_SECONDS (or LON_SECONDS) is non-numeric.
- Error Message: LAT_MINUTES (or LON_MINUTES) must be sixtieths of a degree, in the range 0 to 59.
Further Explanation: All Latitude and Longitude fields exist as numeric only, with specified decimal places that support alternate coordinate methods introduced with EDSA7. See the New Format Table for details related to structure.
Back to Top
Error Message: Lat_Second and Lon_Second must be sixtieths of a minute, in the range 0 to 59.9999. If number represents thousandths of a minute, multiply it by 60/1000 (0.06). If number represents hundredths of a minute, multiply it by 60/100 (0.6)
Error Message: Lat and lon require precision of 0.1 sec or smaller
Explanation: If reporting data in Lat/Longs, remember that there are two significant digits to the right of the decimal point (hundredths of a second) that are enforced in Site Remediation regulations/guidance. These are 0.01 seconds of Latitude/Longitude for surveying wells and 0.01 seconds of Latitude/Longitude for submission to HazSite. Failure to report Latitude or Longitude to the tenth of a second results in an error. One second of latitude at 40 degrees north is approximately 111 feet; one second of longitude is approximately 78 feet at the same latitude. One-tenth of a second of: latitude = 11.1 feet; longitude = 7.8 feet.
Back to Top
Error Message: All latitude and longitude fields must be populated if NJ State Plane Coordinates are not.
Explanation: All latitude and longitude fields must be populated if NJ State Plane Coordinates are not. There is now an absolute requirement for distinct X and Y locations that are submitted in either Latitude and Longitude or State Plane X and Y coordinates. Where both are submitted, they must agree as to the actual location of the sample. For further explanation, see FIELDID XY explanation
- Sp_x, Sp_y
Error Message: Example: Outside Easting range of NAD83 NJ State Plane Coordinates. SP_X = 105296
Error Message: SP_X (Easting) is non-numeric.
Explanation: Easting fields must contain numeric values between 193,000 and 658,000 when transformed from text to numeric; this error indicates that the text contains something other than numbers 0 through 9 or the comma or period characters.
Back to Top
Error Message: Example: Outside Northing range of NAD83 NJ State Plane Coordinates. SP_Y = 962413
Error Message: SP_X (Easting) outside range of NAD 1983 NJ State Plane Coordinates in US survey feet.
Explanation: The range of NAD83 (feet) Northing Coordinates is from 34000 to 920000.
Back to Top
Error Message: Example: Latitude, longitude correspond to Easting, Northing 408259.3, 521649.5, which differ by -12.4 ft in Easting, 32.5 ft in Northing, 34.7 ft overall distance
Explanation: Latitude and longitude (corresponding to Easting and Northing) are checked by EDSA7 to ensure that coordinates are correct. If Latitude and Longitude are submitted along with the X and Y coordinates, the Lat/Longs are converted to State Plane Feet and checked against the submitted X-Y coordinate. If discrepancies are evident, then at least one set of coordinates is incorrect. It is better to report one set of coordinates. NJDEP's preferred format is State Plane Feet (in NAD83). If reporting data in Lat/Longs, remember that there are two significant digits to the right of the decimal point (hundredths of a second). Failure to report Latitude or Longitude to the tenth of a second results in an error. One second of latitude at 40 degrees north is approximately 111 feet; one second of longitude is approximately 78 feet at the same latitude. One-tenth of a second of: latitude = 11.1 feet; longitude = 7.8 feet (the level required for surveying wells in NJ is one-hundredth of a second for latitude and longitude).
For soil borings, an estimate can be made by using the imagery from the site in NJ GeoWeb to estimate X-Y coordinates from the lower right of the main window by "mousing over" the desired location.
Figure 3: Estimating X-Y Coordinates with NJ GeoWeb

A second option for working with coordinates from a single known location is the State Plane Feet Calculator (downloadable as an XL Spreadsheet - see below). This spreadsheet allows the user to input the distance and angle from an origin point and it will calculate the coordinates (in feet) from the origin. These coordinates are sufficient to allow calculation of an acceptable X-Y coordinate for submission to NJDEP.
Figure 4:
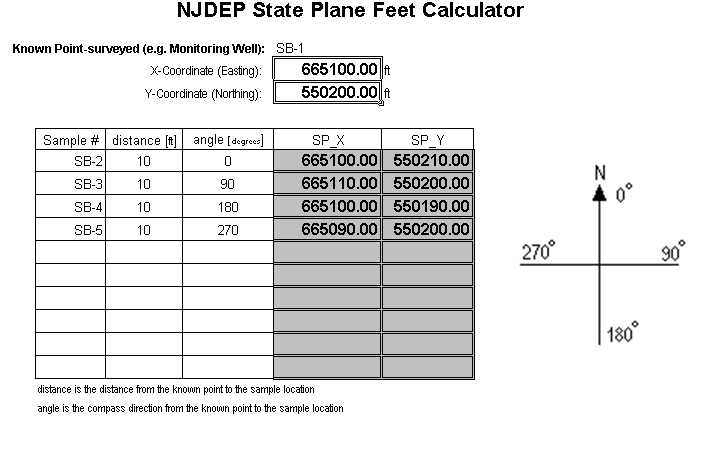
CorpsCon 6.0 Program
This is a latitude-longitude to state plane feet conversion utility program from the U.S Army Corps of Engineers. The Corps site describes the program as a "...program which allows the user to convert coordinates between Geographic, State Plane, Universal Transverse Mercator (UTM) and US National Grid systems on the North American Datum of 1927 (NAD 27), the North American Datum of 1983 (NAD 83) and High Accuracy Reference Networks (HARNs). Corpscon uses the National Geodetic Survey (NGS) program Nadcon to convert between NAD 27, NAD 83 and HARNs."
- Depth_top
Error Message: DEPTH_TOP should not state unit of measure; number must be in feet.
Explanation: DEPTH_TOP should not read 2.5' because feet (represented by ') is assumed to be the required unit of measure.
Back to Top
Error Message: DEPTH_TOP is above expected range. Enter 'N/A' if this required depth is inapplicable to sample or unavailable.
Error Message: DEPTH_TOP is above expected range. Depth is optional for a blank sample or one from a transient soil pile, so 'N/A' or an empty field is acceptable.
Error Message: DEPTH_TOP is below expected range. Enter 'N/A' if this required depth is inapplicable to sample or unavailable.
Explanation: The DEPTH_TOP is structured on an expectation that it would not be necessary to exceed 6 characters (Max depth = 999.99 feet) for any sample collected for the HazSite data collection process. An error would be generated for any sample with a depth greater than or equal to 1,000 feet below the ground surface. If this entry is invalid, it is acceptable to enter "N/A" or leave the field blank.
Back to Top
Error Message: DEPTH_TOP is below expected range. Depth is optional for a blank sample or one from a transient soil pile, so 'N/A' or an empty field is acceptable.
Explanation: DEPTH_TOP should not normally be less than zero (since 0 is acceptable as an entry). Some types of samples, notably temporary soil piles, could be considered to be above the ground surface, therefore, the relation to "below ground surface" would be, say -3 for a sample collected from a one-foot depth into a four-foot high soil pile. For this reason, this error message is considered a warning and will not cause a dataset to fail. NJDEP does not, however, require depths from transient soil piles that will be removed in a timely manner and thus it is acceptable to leave the field blank or use "N/A".
Back to Top
Error Message: DEPTH_TOP is empty.
Explanation: DEPTH_TOP is required for soil samples and for ground water samples. It is not acceptable to leave the field blank in these cases.
Back to Top
Error Message: DEPTH_TOP is invalid. Enter 'N/A' if depth is not applicable or not available for sample.
Error Message: DEPTH_TOP is invalid. Enter 'N/A' if depth is inapplicable or unavailable for sample.
Explanation: The DEPTH_TOP is sturctured on an expectation that it would not be necessary to exceed 6 characters (Max depth = 999.99 feet). Should one encounter an error that was not specifically addressed in prior explanations, one of the two messages above may be issued.
Back to Top
Error Message: DEPTH_TOP exceeds the 6-character limit
Explanation: The DEPTH_TOP is limited to 6 characters. The practical implications of this are to limit the sampling depth to a range from 0 to 999.99 feet below the ground surface.
- Depth_botm
Error Message: DEPTH_BOTTOM - DEPTH_TOP > 0.5 ft. Is unit of measure < 1 foot?
Explanation: The DEPTH_TOP is compared to DEPTH_BOTM for each sample collected. Occasionally, depths have been entered incorrectly. The most common is to use measurements in inches. Doing so might result in seeing something like: DEPTH_TOP = 12 and DEPTH_BOTM = 18. If these are inches (1.0 - 1.5 feet below ground surface) this makes sense, but NJDEP normally requires sampling cores to be no more than 2 feet in length and samples from the core to be biased to the six-inch interval with the highest probability for contamination. Use of DEPTH_TOP = 12 and DEPTH_BOTM = 18 makes the sample appear to have been from a 6-foot core, which would be invalid.
Back to Top
Error Message: DEPTH_BOTM less than DEPTH_TOP. If DEPTH_BOTM is unknown, empty or Null is acceptable for this non-mandatory value.
Explanation: DEPTH_BOTM is only required for soil samples, therefore it should never have a zero and inputting a zero for soil samples will result in EDSA7 evaluating this field as an error since it will be less than any non-zero DEPTH_TOP. Leave this field empty (null) for a sample that does not require a bottom depth.
Back to Top
Error Message: DEPTH_BOTM should not state unit of measure; number must be in feet.
Explanation: DEPTH_BOTM should not read 2.5' because feet (represented by ') is assumed to be the required unit of measure.
Back to Top
Error Message: DEPTH_BOTM is above expected range. Enter 'N/A' if this depth is inapplicable or unavailable for sample.
Error Message: DEPTH_BOTM is below expected range. Enter 'N/A' if this depth is inapplicable or unavailable for sample.
Error Message: DEPTH_BOTM is invalid. Enter 'N/A' if this depth is inapplicable or unavailable for sample.
Error Message: DEPTH_BOTM exceeds 6-character limit.
Explanation: The DEPTH_BOTM is structured on an expectation that it would not be necessary to exceed 6 characters (Max depth = 999999 feet) for any sample collected for the HazSite data collection process. An error would be generated for any sample with a depth greater than or equal to 100,000 feet below the ground surface. If this entry is invalid, it is acceptable to enter "N/A" or leave the field blank.
Back to Top
Error Message: Required DEPTH_BOTM is empty or invalid. Enter 'N/A' if not applicable to sample.
Explanation: The DEPTH_BOTM is required for soil samples only. If the particular sample does not require a bottom depth input, then the field cannot be empty and should be filled with "N/A"
- GroundElev
Error Message: Invalid mandatory GROUNDELEV
Explanation: The GROUNDELEV text field does not evaluate to a number or the number exceeds 1,803 feet above mean sea level.
Back to Top
Error Message: GROUNDELEV exceeds 6-character limit
Explanation: If a number (such as 1003.66) is entered into the field, the size of the field would be exceeded. Although the number is valid, the field holds only 6 characters (including the decimal point).
- Well_elev
WELL_ELEV The Well Elevation is a value for the Z-Coordinate, measured in feet above mean sea level (MSL), for the location of the surveyor's mark on the well casing. This measurement should be surveyed to the North American Vertical Datum (NAVD) 1988, to an accuracy of 0.2 feet, using generally accepted surveying methods.
Error Message: Invalid WELL_ELEV
Explanation: The WELL_ELEV text field does not evaluate to a number or the number exceeds 1,809 feet above mean sea level.
Back to Top
Error Message: WELL_ELEV exceeds 6-character limit"
Explanation: If a number (such as 1006.66) is entered into the field, the size of the field would be exceeded. Although the number is valid, the field holds only 6 characters (including the decimal point).
- Datetolab
See Date Errors
- Wellpermit
Error Message: Required WELLPERMIT is empty.
Explanation: This is now a required field. If the well is unpermitted or grandfathered and does not require a permit use "NO PERMIT" in this field. Any other text will cause an error. All wells except for domestic wells per-dating 1949 should have a valid permit. Well permits are formatted according to a system that uses the Atlas grid designation for the first two characters. The acceptable numbers are: 21, 22, 23, 24 , 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 and 37. Following the two characters, will be a minimum of three zeros and finally five or fewer digits that are a sequential numbering system for the wells permitted for that Atlas Grid sheet.
In other words, there are two numerical characters signifying the atlas grid sheet at the beginning of the string and up to five numerical characters that designate the well number for that sheet. Between these two numbers, the string is padded with zeros to make a 10-character permit number
In the old system a hyphen was used in the place of the padding zeros. If we compare numbers from the old system to the new system, they might look like:
Old: 31-17647
New: 3100017647
If a permit number is encountered with a second hyphen and additional numbers, ignore everything from the second hyphen on for the purposes of the well permit. Permits were often issued for multiple wells and the final numeric portion helped drillers track which well was which in submitting a single permit for multiple wells. The well record number ultimately became the permit number when the well was recorded in the database and the permit information will show up for all relevant wells.
Starting around 2008, wells were submitted electronically (or keyed in from paper copies) to the Department's NJEMS system. Those wells were required to have accurate X-Y coordinates and no longer rely on the Atlas Grid system. Their format begins with a letter, P for Paper; E for Electronic. This is followed by the year (e.g. 2009) and a sequential number of up to 5 numeric characters (allowing for up to 99,999 wells to be drilled within one year for the state). The unused portion of the 5-digit number is filled with zeros, as before. The well permit number might look like "P200905860".
In short, all well permit numbers now have 10 characters. If there
Error Message: WELLPERMIT exceeds 10-character limit
This is a new field and is a required field. Evaluation of data has been hampered by lack of proper coordinates. NJDEP has decided to require this field in order to evaluate the actual location of wells being sampled as part of site investigations/cleanups.
The format of the well permit was previously based on the Atlas Grid system. The first two digits of the permit represented the Atlas Grid sheet in which the well was located (Note that in the image below the first 2 digits in the Application Number are the same as the first two digits in the Location, or Atlas Grid). The remaining digits represented the sequential number for the well drilled in that Atlas Grid. NJDEP eventually found it necessary to "Normalize" the data for wells to help with lookups of wells when the paper information was entered into databases. To do so, the well had "leading" zeros placed in front of the well number.
Originally, normalized wells were stored as Atlas Grid + hyphen + Well Number (26-01335, for instance). In 2008, the well program moved all of the wells into the New Jersey Environmental Management System (NJEMS)and again normalized wells to a 10-character format. Wells from the prior system were "padded" again with leading zeros, resulting in the Atlas Grid + leading zeros + the well number. The prior number (26-01335) would be expressed as 2600001335 in the new system.
Any newer wells were input directly into NJEMS by NJDEP staff from paper or by the driller through an internet connection via the Remedial Services Portal (RSP). Wells that were input in this way had a completely new designation. The first character was either P (for Paper) or E (for Electronic) followed by the 4-digit year and a 5-digit sequential number for the well. Thus the first electronic well of 2009 would have the designation "E200900001" and so forth.
Figure 5:

HZRESULT
- Sampdate
See Date Errors
- Labid
Error Message: LABID exceeds 20-character width.
Explanation: The maximum length for this field is 20 characters. Any requirement for truncation (shortening) of this field should take into account the other LABIDs in the dataset. One of the most common errors is that exceedingly long strings are created. When they are placed in a field with insufficient length, most computer programs remove characters from the right side of the string. If part of the string is a Numeric string or one that has to do with dates, this will likely result in creation of duplicates.
Example: The first LABID is GC#4_20110301-1300:09:06:01; the second LABID is GC#4_20110301-1300:09:07:15 (note - the first 20 characters are in bold-underline type).
Further Explanation: Both LABIDs would be truncated to GC#4_20110301-1300:0 (if characters at the right of the string are removed, while LABID 1 would be: 110301-1300:09:06:01 and LABID 2 would be: 110301-1300:09:07:15 if truncated from the left.
- Labname
Error Message: LABNAME exceeds 20-character width.
Explanation: The Lab Name must be no more than 20 characters. Lab Names may be truncated or abbreviated if necessary. The key field for identifying a laboratory is the NJDLABCERT field (see below).
- Njdlabcert
Error Message: NJDLABCERT Exceeds 7-character width
Explanation: This field may not exceed 7 characters. If you require additional assistance related to certified laboratories, please click on the link below:
Back to Top
Error Message: NJDLABCERT is empty
Explanation: NJDLABCERT cannot be empty - it is a required field
Data Miner Reports for Certified Labs
- Resulttype
Error Message: RESULTTYPE Exceeds 1-character width
Error Message: RESULTTYPE is empty
Explanation: This field cannot be empty and has a limited set of acceptable valid values. These valid values are listed in the table below and explained in detail below the table.
- Conc
Error Message: Required CONC is empty.
Explanation: CONC may not be left empty. If there is no value, use 0. The detection limit will be obtained from the MDL field of from the combination of the QUANTTYPE and QUANTLEVEL fields.
Back to Top
Error Message: Invalid value for CONC
Explanation: The CONC field requires a text expression that evaluates to a valid numeric expression when the value of the field is assessed. Any field using characters other than 0 through 9 and the decimal point will generate an invalid entry and thus, an error.
Back to Top
Error Message: CONC exceeds 12-character limit
Explanation: The CONC field is limited to 12 characters. This should allow entries between .00000000001 and 999,999,999,999 since the decimal point is not required (commas are not required but included in this explanation for ease of understanding).
- Anlys_mthd
Error Message: Required ANLYS_MTHD lacks 4-digit method number (or chapter ref) for SW-846 method.
Explanation: Please see EPA's Solid Waste - 846 references for details.
Back to Top
Error Message: Required ANLYS_MTHD contains no method number.
Explanation: Each method consists of a text prefix followed by an alpha-numeric expression (for example in "SW8468260B" SW=Solid Waste 846 is the EPA identifying number for the publication and 8260B is the method)
Back to Top
Error Message: Required ANLYS_MTHD is empty.
Explanation: The field cannot be left empty. If no appropriate entry is available for this field, put N/A in the field.
Back to Top
- Uncor_conc
Error Message: Required Uncor_Conc is empty
Explanation: This newly-required field is mandatory for air sampling.
- Uncor_unit
Error Message: Required Uncor_Unit is empty
Explanation: This newly-required field is mandatory for air sampling.
- Reten_time
Error Message: Retention time of this TIC should appear at end of AnaltParam field OR at end of CAS field OR in recently added 22nd field Reten_Time.
Explanation: Tentatively Identified Compounds (TICs) are now required to have information related to their respective retention times within the column on which the analysis was conducted.
- Danalyz
See Date Errors
Back to Top








